
N95 respirators are medical devices; learn how FDA treats these devices that are critical to our nation’s COVID-19 response.
- Posted by Melissa Nickel
- On March 20, 2020
Protective masks like the N95 are in the news with shortages, stockpiles and new legislation around this important personal protective equipment. In this article we summarize how the FDA regulates these products, who makes them and what’s in place to make sure that they work!
There are generally two categories of face masks being discussed: N95 respirators and surgical (face) masks. As published by JAMA (The Journal of America Medical Association), here is the difference: “Face masks fit more loosely and prevent the wearer from spreading large sprays and droplets when coughing or sneezing. N95 respirators fit more tightly and prevent the wearer from inhaling smaller, airborne infectious particles.”
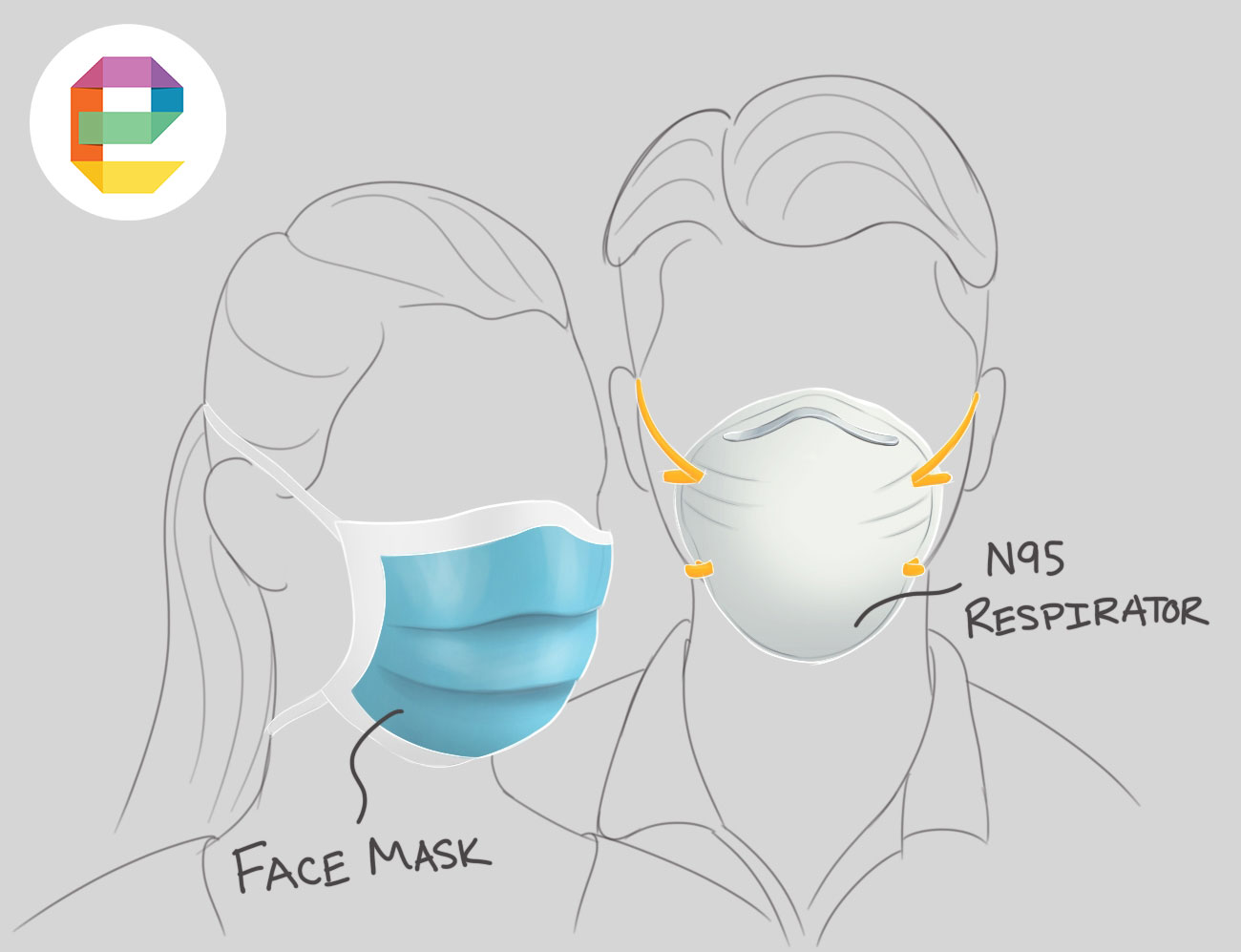
In the United States, N95 respirators are considered class II medical devices by the Food & Drug Administration. Class II is designated to have a moderate risk of harm to the patient and/or user (examples of other class II devices include powered wheelchairs and pregnancy test kits). According to the FDA, 53% of medical devices are in the class II category. For perspective, the next step up, class III devices, are considered high risk and include devices that “sustain or support life, are implanted or present potential high risk of illness or injury”.
A medical device can be approved through the 510(k) process if it is shown by the manufacturer to be substantially equivalent to a product already on the market. In addition, the FDA requires that manufacturers meet certain performance standards. Under normal circumstances, a manufacturer is required to register with the FDA (for their device and organization) and production lines must also be registered with the FDA for the production of a medical device. Registered production facilities are subject to FDA inspection and surveillance – important steps to keep the public safe.
For more information on this important topic, the FDA has a summary of how N95 respirators are intended to be used. Also look at the Center for Disease Control & Prevention’s list of approved manufacturers.
ABOUT THE AUTHOR(S)
Melissa focuses on all aspects of business operations at Engenious Design. She is a strategic problem solver with a sense of urgency, productivity and positivity. Melissa’s passion for pursuing innovative solutions pairs well with her background in product development project management and current position coordinating collaborative teams of expert designers and engineers working on medical devices and advanced technology products.




