Get your embedded software done faster.
Engenious is a design firm built to help R&D leaders who have more projects than people.
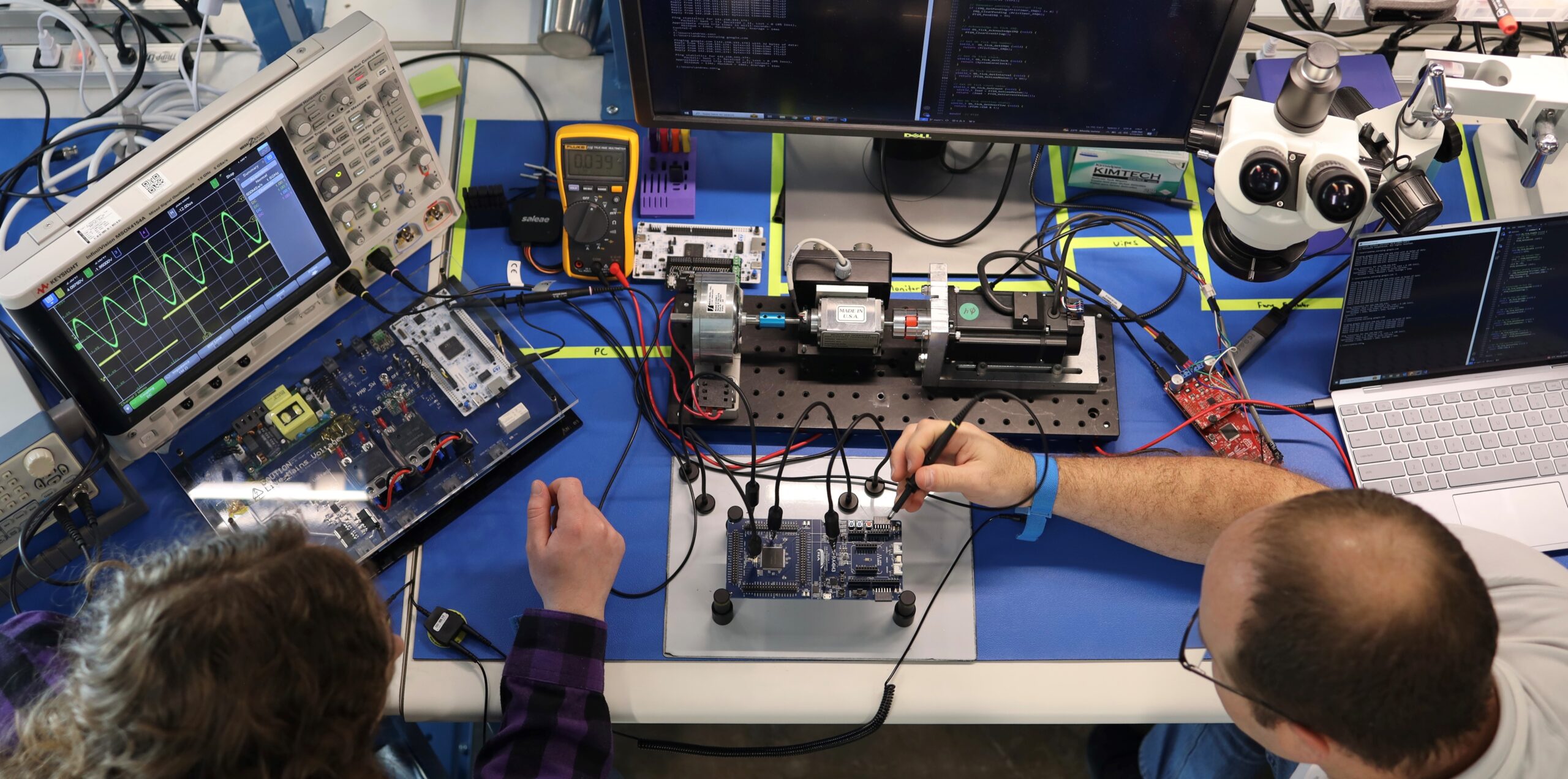
There’s a reason other top medical device R&D leaders turn to Engenious when they have more projects than people. Our team is highly-experienced with embedded software design for medical devices. We bring you one of the largest domestic USA teams, at 75 people and growing. Our experience and team size means we can move faster for clients and get projects across the finish line more quickly than others.
We know Embedded Linux better.
You don’t have time for novices; and you need experienced help now. Our team has active involvement in the Linux community for over 20 years. In fact, other engineering teams and even Linux experts come to Engenious when it’s time to modify the Kernel or solve challenging problems. When competitor design firms need embedded Linux help, they turn to Engenious too. We consider that a big vote of confidence.
UI Design
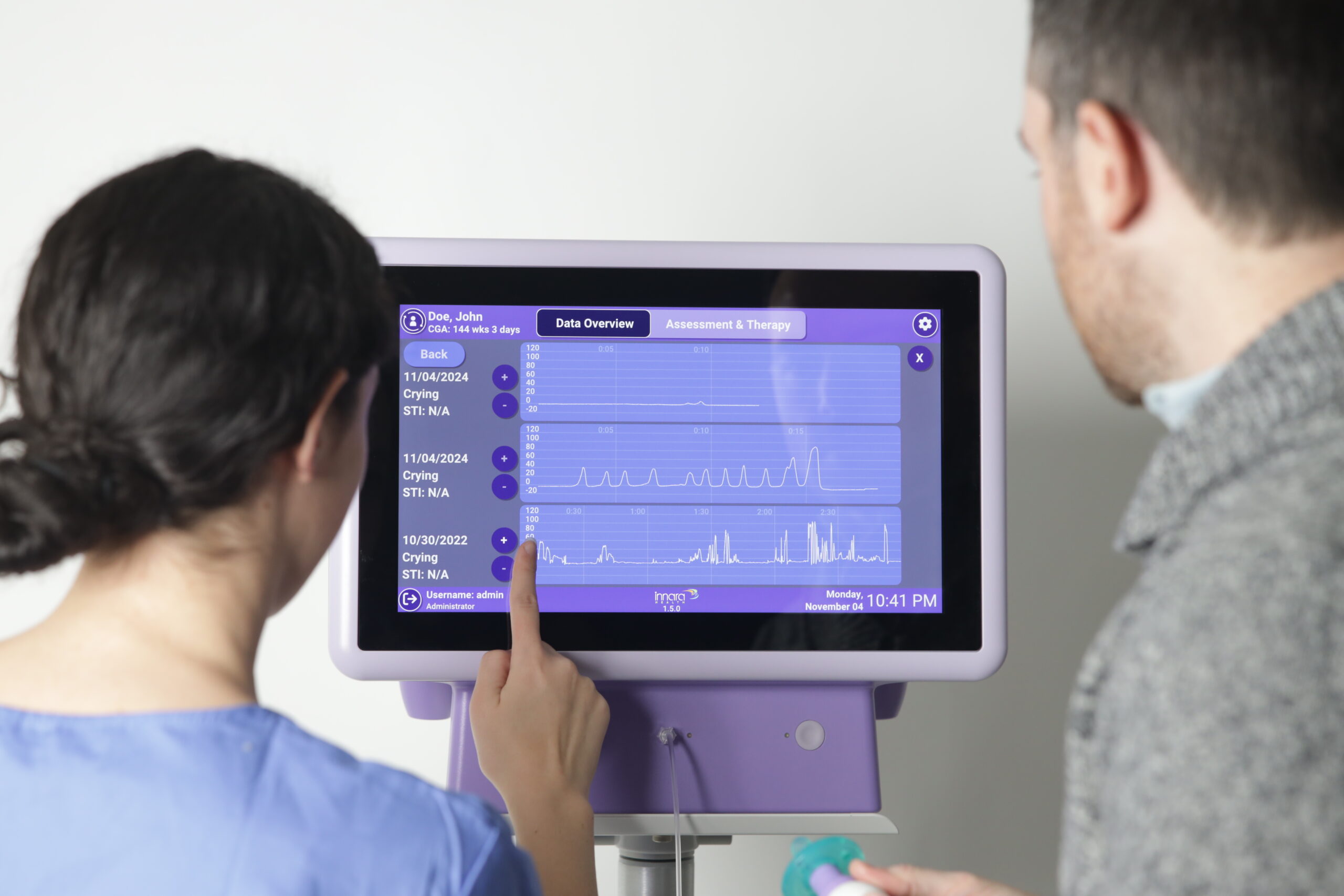
You need a system that’s intuitive to use, but also one that fits into clinical workflows. This is why our software team works directly with our in-house UI design team. Our design team is expert at quickly understanding who users are, key use cases, workflows, use hazards and opportunities to innovate.
To support you with clinical insights, we have assembled a large and growing group of clinicians available to provide design input. We quickly find out about competitor systems, points of pain and unmet needs because we do in-person clinical observation visits to inform design. We bring clinicians into the design process early, so design stays clinician-focused.
From wireframes to pixel-accurate mockups to final design, our team has all the tools to make modern user interfaces that are a joy to use. Because most of the systems we develop are targeted at international distribution, our team knows how to design and validate multi-language user interfaces that fit anywhere in the world.
Real-time Software
If you are leading medical device R&D, you might need real-time help. Not every system needs embedded Linux, and we have experience developing with real time operating systems. Sometimes it’s for motion control or custom motor controllers. Or we might develop software to control breath delivery in a ventilator, drug delivery in an infusion pump, or RF energy delivery for electrosurgery systems.
If your application needs real time help, Engenious Design has the experience to get your system moving quickly. Our team knows how to design and tune your PID systems because we do it every day for critical care devices.
Human Factors and Usability Engineering
You need to know your users. So we plan, organize and conduct field research in clinic. Our team is in the OR regularly, and does user research in all areas of in-clinic and homecare settings. And our
Our team works with FDA guidance on human factors. And we also work to IEC:62366 – the standard for medical device usability engineering. We can plan and conduct your next usability study, from early formative work to final summative validation.
Today's Cybersecurity
As an R&D leader, you may not have time to keep up on Cybersecurity regulations which move quickly. And the threats move even more quickly. Engenious invests to stay on the cutting edge of security. This means we create design outputs that are ready for regulatory scrutiny and fast clearance. And also ready to be secure in the real world to ensure confidentiality, integrity, and availability.
A key part of security is threat modeling, and Engenious helps clients build and maintain threat models. We create architecture views to consider how attackers could exploit system weaknesses, the severity of an exploit and motivation for attack. Then provide balanced security mitigations for these threats. Because we work with many clients, we don’t need to start from zero with every threat model; we can build on our experience for a jump start to comprehensive threat modeling.
Security risk analyses are important (and required) in modern medical devices. And connected medical devices are increasingly complex, exposing risks to every part of a system. Our systematic approach to security risk analysis means that the right protections are designed in, holding the tension of efficient design and security to protect both you and your clients.
Medical device process natives
You don’t have time for someone to learn about medical device process; you need experience now. And we create medical devices every day, and have been doing so for over a decade. Other teams “can” do medical devices, but it’s the focus of what we do at Engenious and not just something we do every once-in-a-while.
This means we understand FDA guidance for software and cybersecurity, risk management and validation. And our baseline is compliance with ISO:62304 process – the standard for medical device software. So you don’t face documentation issues that delay product clearance.
We most regularly design systems with safety classifications C (the highest level). But we operate on products in all three categories (A/B/C). You won’t need to educate our team on medical device software process, and you can expect software that is ready for fast clearance.
Hardware Foundation
If your design requires electronics, we have you covered. We develop systems that operate on SOMs. SOM’s (System On Module) are pre-developed hardware platforms that can be bough off-the-shelf. But we have a full team of Electrical Engineers who can create full-custom electronics for clients who need more control of design or supply chain.
Our Electrical Engineering team is experienced designing with high-speed multicore processors, DDR memory busses, and other high speed peripherals – and full control over supply chains and technology choices.
Our Embedded Software team works closely with our Electrical Engineering team, who creates anything from the hardware to run multiple displays, precision analog instrumentation, signal acquisition, and motion/motor control.
Our in-house Electronics lab allows us to design, build and test embedded systems faster for our clients. And our in-house labs.
Systems Engineering
Because most of our work is with complex systems, we have an experienced systems engineering/product architecture team. We know how to manage requirements through design and test – and understand modern requirements management tools.
Risk Management
Your medical device project needs a team that knows risk management. Our team has been operating in ISO:14971 – the medical device risk management standard – for decades. We know the latest standard well, and how to meet the letter and intent of this important standard.
Our software and systems teams regularly plan and execute every aspect of a risk management program. We also plug into an existing risk management plan and operate within a client’s quality system.
Our work with so many top medical device manufacturers means we have seen and learned from some of the best practices out there – knowledge we bring into every new program.
Great teamwork starts with a conversation!