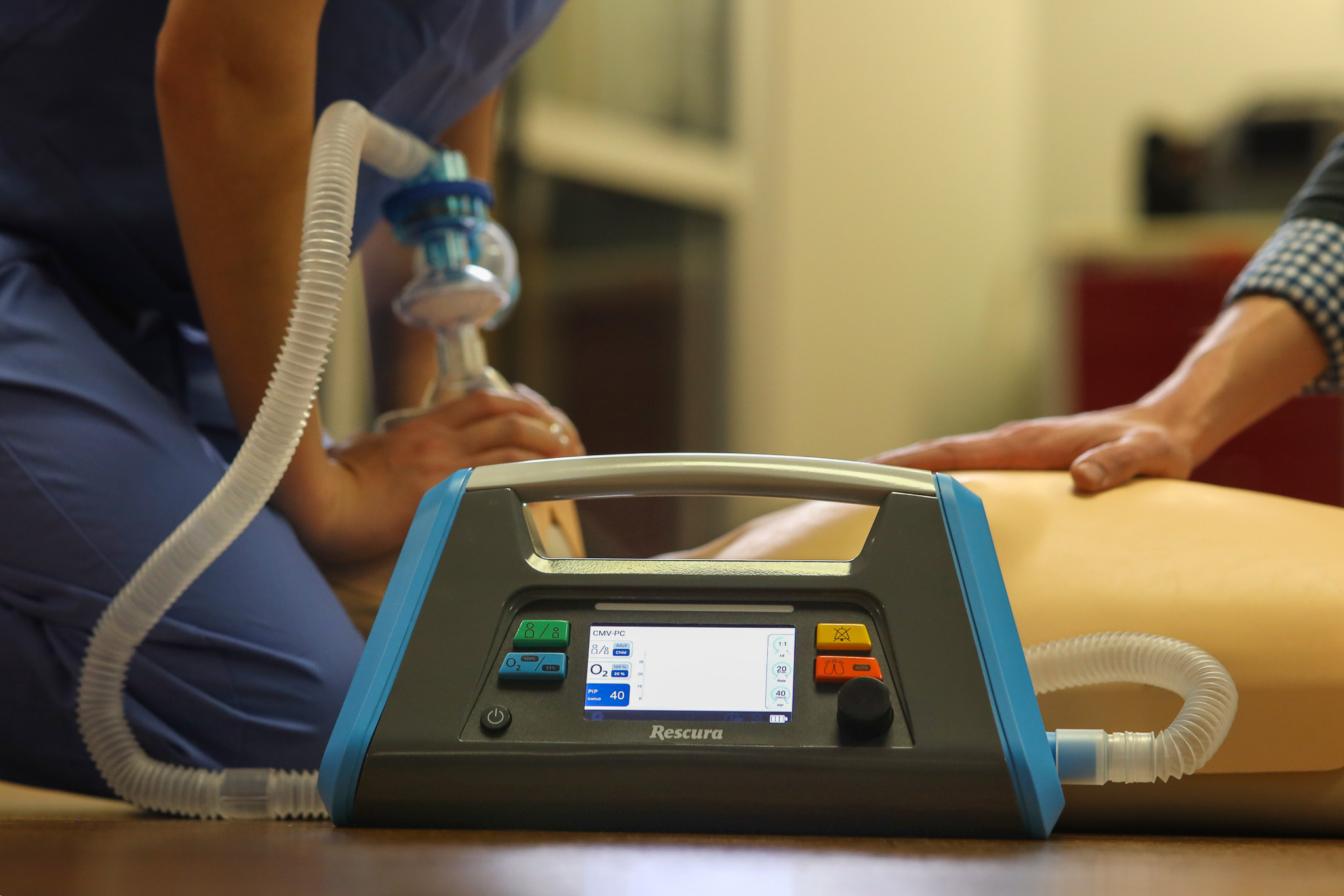
The Rescura system is an emergency automatic resuscitator. This highly portable and simple to use ventilatory provides lifesaving oxygen safely and effectively even with unskilled operators. Engenious developed this device from concept to fully working prototype in just three months. This project involved every part of our team from industrial design to electrical engineering and embedded software.
Our in-house prototyping lab was critical in producing a fully working appearance model that was used to validate the market and test the concept with clinical users. While the models are not approved for sale, they were designed under the same controls needed for eventual certification. We move quickly but with precision and coordination to reduce risk and validate concepts early for clinical success in the long run.
Precision Microwave was founded to bring an amazing and innovative microwave ablation technology to market. The novel approach to precision-controlled tumor ablation had the potential to give surgeons a powerful new tool to fight cancer. The solo founder knew that they needed the help and resources to move from the university lab to full trials and FDA certification.
Three months and about $200k later, Precision Microwave had a functional demo unit designed and built completely in-house at Engenious. We moved faster than anyone thought possible and delivered a prototype system to begin animal lab testing. The system included a full enclosure design, functional components, and a custom, embedded Linux-based system to control treatment and the UI.
.JPG)
Innara came to Engenious with the idea for a research-based product to help premature infants learn to feed on their own. The NTrainer helps clinicians quantify neurological development and provides therapy to encourage a healthy suckle reflex. The result is an embedded Linux system driving a fully customized hardware package. The system was architected to meet FDA and cyber security requirements.
Our team guided the client toward a solution that was effective, compelling and ultimately attractive for acquisition. The product concept was ultimately acquired by a large, international medical device company who worked with Engenious through the certification process and successfully launched into the neonatal market.
.JPG)
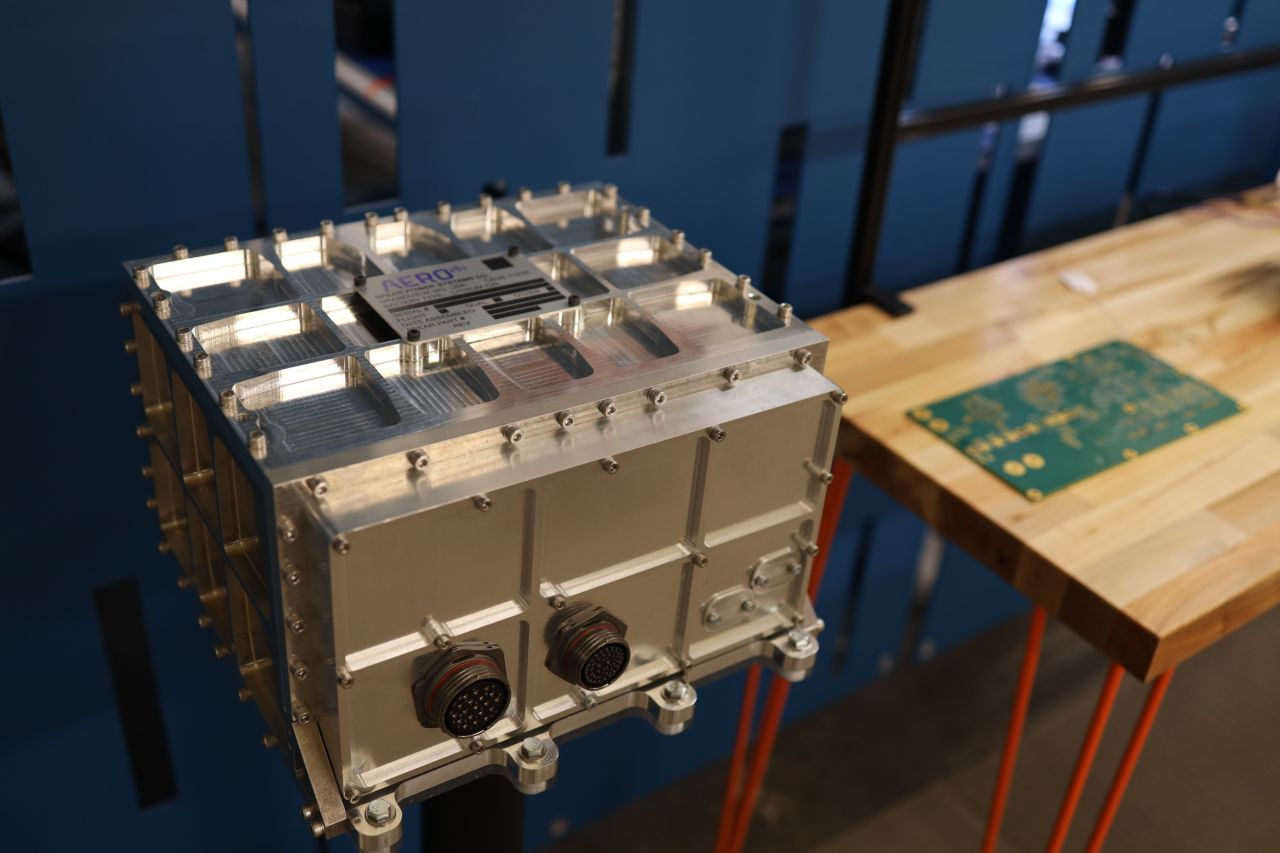
At Engenious, we understand that the work we do can be the difference between life and death. Reliability is built on strong processes and design controls that support the world-class engineers creating solutions for our clients. So, when Blue Origin needed help developing a mission-critical power management component, they turned to Engenious.
Our team of electrical engineers worked side-by-side with a component supplier to develop a custom power management system that delivers power to core flight control systems for the New Glenn rocket. The Engenious in-house test team provided validation of performance requirements to ensure that our design was ready for the rigors of space flight. Because with a 322-foot rocket making 3.9 million pounds of thrust traveling at 17,000 mph, reliability is not optional.
BD approached Engenious to help de-risk a device acquisition and ended up revolutionizing the incontinence market. Through numerous focus groups, user interviews, and in-home visits, we were able to define the product form in a way that provides a vastly improved quality of life to patients and caregivers. These highly emotional and personal interactions allowed us to gather critical design input and nuanced feature feedback that shaped the development and, ultimately, the manufacturing of the product.
DryDoc is a wearable external female catheter combined with a quiet suction pump that removes urine quickly and discreetly. This frees patients from the discomfort and embarrassment of traditional incontinence solutions. The design features a reliable and quiet pump, an optimized slimline enclosure, whisper-quiet sound control, and vibration dampening—all while exceeding the performance of the original design.
Ultimately, we delivered an updated design with low Cost of Goods Sold (COGS), high reliability, and an intuitive user experience. Through extensive Design for Manufacturing (DFM) work, we were able to reduce the overall part count and simplify the assembly process. We proved the effectiveness of our DFM work by producing a 100-unit pilot run in-house for user testing. Today, the BD DryDoc and PureWick are known as the market leaders, trusted by tens of thousands of users.
